Outbreak of Salmonella Serotype Enteritidis Infections Associated with Raw Almonds — United States and Canada, 2003–2004
On June 4, this report was posted as an
MMWR Dispatch on the MMWR website:
(http://www.cdc.gov/mmwr).
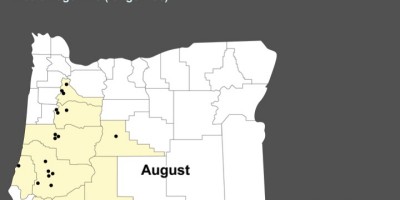
On May 12, 2004, the Oregon State Public Health Laboratory identified a cluster of five patients infected with Salmonella enterica serotype Enteritidis (SE) isolates that were matched by using two-enzyme pulsed-field gel electrophoresis (PFGE). The five patients were from four Oregon counties; their onsets of illness occurred during February–April 2004. A subsequent investigation, still ongoing, has identified a total of 29 patients in 12 states and Canada with matching SE isolates, since at least September 2003. Seven patients have been hospitalized; no one has died. Raw almonds distributed throughout the United States and internationally have been implicated as the source of the SE infections. As of May 21, approximately 13 million pounds of raw almonds had been recalled by the producer.
Routine interviews of the initial five patients with salmonellosis had not indicated a common exposure. However, prompted by the May 12 laboratory data, the patients were reinterviewed by using a standard hypothesis–generating questionnaire that included questions about consumption of approximately 400 specific food items and their shopping and eating venues during the 5 days before illness onset. Using binomial distribution, consumption rates for selected foods were compared with background rates estimated from a 2002– 2003 population-based survey of residents of Oregon (1). The initial five patients from Oregon all reported consuming Kirkland Signature brand raw almonds, purchased at Costco warehouse stores. Survey data (1) indicated that an estimated 9% of Oregon residents (86 of 921 surveyed) consumed raw almonds from any source in the preceding week. Even assuming that 20% of all Oregon residents ate Kirkland Signature brand raw almonds each week, the binomial probability of finding five of five sporadic cases with that history is <0.001. No other foods or food sources were associated with illness.
After determining that the raw almonds were distributed widely, U.S. and Canadian epidemiologists and state and federal regulatory agencies were notified on May 13 via electronic information networks. Through PulseNet, the national molecular subtyping network (2), laboratories were queried for reports of isolates matching the outbreak PFGE patterns (XbaI: JEGX01.0049; BlnI: JEGA26.0008 or JEGA26.0009, reflecting minor variation later observed with the second enzyme). Laboratories that did not routinely screen SE isolates by using PFGE were encouraged to do so for isolates collected since February 1, 2004. Phage typing was performed by standard methods. As additional PFGE-matching isolates were identified, a brief, customized questionnaire was used in interviews with persons about their nut consumption.
Raw almonds from an opened package recovered from one patient’s household were tested for Salmonella by enzyme immunoassay. Unopened packages of nuts from the supplier’s warehouse and environmental samples collected at the almond processor and at huller-shellers supplying the processor were tested for Salmonella by using standard microbiologic methods.
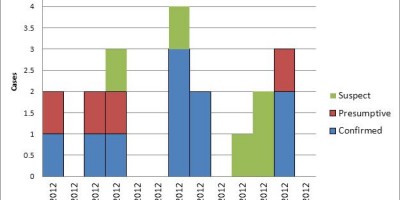
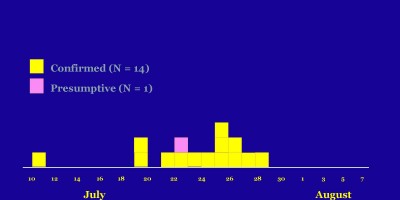
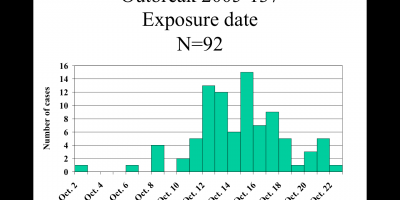
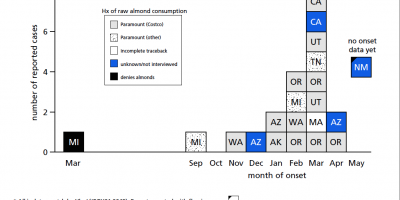
As of June 2, a total of 29 patients with SE infections matching both XbaI and BlnI PFGE patterns had been identified in 12 states and one Canadian province. Symptom onsets ranged from September 2003 to April 2004 (Figure). Patients ranged in age from 11 months to 91 years (median: 40 years); 17 (59%) were female. Seven patients were hospitalized; no one died. Multiple other cases with matching PFGE patterns and onsets earlier in 2003 remain under review. To date, nine isolates from the
current outbreak have been phage typed; all are type 9c, which is uncommon. Among 26 patients interviewed, 24 recalled eating raw almonds during the week before illness onset; 20 patients identified brands packaged or supplied by Paramount Farms (Lost Hills, California). One infant patient was presumed secondarily infected. Through retailer computer records linked to membership cards or customer receipts, dates and places of almond purchase were verified for 10 households of patients. The dates of verified almond purchases ranged from November 3, 2003, to January 28, 2004.
Efforts to identify specific production lots associated with illness, based on almond purchase dates and locations and store inventory data, are ongoing. On May 18, Paramount announced a nationwide recall* of all raw almonds sold under the Kirkland Signature, Trader Joe’s, and Sunkist labels. Costco mailed 1,107,552 letters to members known to have purchased the recalled product in the United States. The recall was expanded subsequently to include nuts sold in bulk to approximately 50 other commercial customers, some of whom repackaged almonds for sale under other brand names. In addition to sales in the United States, almonds were exported to France, Italy, Japan, Korea, Malaysia, Mexico, Taiwan, the United Kingdom. The majority of the recalled almonds likely were consumed months ago; however, raw almonds have a shelf life of >1 year, and consumers might still have the implicated products.
Tests of raw almonds recovered from a patient’s household and samples collected at Paramount were negative for Salmonella; however, Salmonella was isolated from one environmental sample collected at Paramount and from three samples from two huller-shellers that supplied Paramount during the period of interest. Serotype and PFGE analyses of these isolates have not been completed, and additional sampling continues. |